Engineer Review Customer Requirements for System Implementation Project Plan
An ISO 9001 Projection Plan volition give you an overview of the steps required for achieving certification as well every bit a ameliorate understanding of the scope of the project.
Nigh ISO 9001 projects accept 3 to 12 months.
| First month | 1. Discover out about ISO 9001:2015 two. Do a gap analysis 3. Get the bankroll of Pinnacle Management 4. Develop your project plan five. Define the Quality Policy half dozen. Ascertain the Quality Objectives 7. Get-go your Quality Transmission |
|---|---|
| 1 - 3 months of keeping records | eight. Certificate the mandatory procedures nine. Select internal auditors 10. Train internal auditors 11. Implement the Quality Management Organization (QMS) 12. Select Certification Torso 13. Refine the QMS 14. Direction review xv. Implement any system changes |
| Final calendar month | 16. Certification Torso preliminaries 17. Certification Twenty-four hours 18. Maintain and improve your QMS |
Although y'all can certainly speed up the process and exercise it quicker, most certification bodies wish to see at least 3 months of record history before their audit.
Don't Try To Manage Information technology All Lone!
Our ISO 9001 Project Plan Template is proven to piece of work.

1. Find out about ISO 9001:2015
Purchase a re-create of the ISO 9001:2015 standard - this is essential!
Start learning almost ISO 9001:
- The ten ISO 9001 clauses
- The mandatory documents & records
Learn nigh the Ten ISO 9001 clauses
Certification and Accreditation data
Generally, Certification Bodies inspect small companies for 1 day, once per yr. Bigger organizations are audited twice per year. To go information on potential auditors please encounter:
- www.iso.org/iso/en/info/ISODirectory/countries.html
- www.ukas.org
NOTE: you lot are audited and "certified" by Certification Bodies; Accreditation Bodies audit and "accredit" the certification bodies.
2. Do a Gap Analysis
The purpose of this gap analysis is to place the areas in your company that require change in gild to exist compliant.
Our Quality Manual Template includes a 18-folio gap assay template which:
- presents an overview of the requirements of each section
- allows space for findings and noting implementation actions
- questions are phrased to permit one give-and-take answers (yeah/no)
Why should I start with a gap assay?
A gap analysis volition compare your current systems with the requirements of the standard. Where in that location is a shortfall, it is called a "gap". This will show the processes:
- that are not currently in place
- that do not comply with the standard and must exist redesigned
- that comply with the standard and must be documented
- that comply with the standard and are documented
The gap assay volition give Top Management a steer as to the scope of try and resources needed to gain certification.
3. Go the backing of Height Management
Every sub-clause of ISO 9001 Section 5, Direction Responsibility, begins with the phrase, "Tiptop Direction shall..."
Pinnacle Direction commitment is essential. Make sure they understand they must provide bear witness of their commitment to developing and implementing the Quality Direction System, as well every bit continually improving its effectiveness.
Unsure they empathize they will exist interviewed by the Internal Auditor and the Certification Body Auditor.
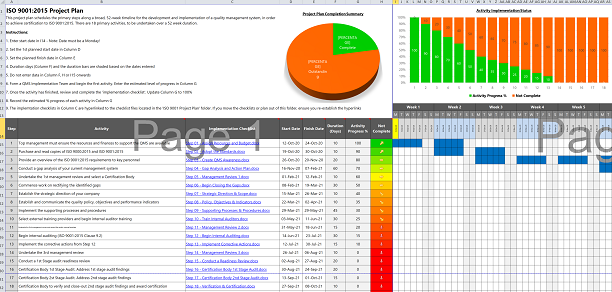
4. Develop your projection programme
Develop your projection plan based on your gap analysis and determine how much or how little documentation you need to demonstrate command.
First with Proficient Templates, then Make Them Yours
Our ISO 9001 Project Plan Template is proven to work.
5. Ascertain the Quality Policy
Information technology's important the Quality Policy is defined by Height Management.
The reason y'all need to define 'quality' is simply that, if you lot don't know what it is, you'll never know whether or not you are achieving information technology.
Not knowing where y'all want to go to also makes it difficult to communicate to other people what is to be accomplished and why, let lonely to motivate them to act.
6. Ascertain the Quality Objectives
Your must define your Quality Objectives. These must reflect the quality policy, be coherent, and align with the overall business objectives, including customer expectations.

7. Start your Quality Manual
Use our Quality Manual Template to assistance document your Quality Management Organisation (QMS).
View Quality Transmission PDF sample.
viii. Plant your Documented Procedures
Use the outputs of your gap analysis to document your processes. These generally include:
- Documented Data
- Risks & Opportunities
- nonconformity & Corrective Action
- Client Satisfaction
- Internal Auditing
- Management Reviews
9. Select Internal Auditors
Internal Auditors should exist people chosen from across the organisation that are inquisitive and open-minded.
Most certification bodies require at to the lowest degree three months history betwixt the formal implementation appointment of the QMS and the certification audit.
Typically, they require that at to the lowest degree one internal audit covering all elements of the QMS are completed and followed past a management review before the certification inspect. This enables the visitor itself to identify problems and to resolve them prior to assessment by the certification body.
x. Train Internal Auditors
The internal auditors need to sympathize how the clause structure and requirements will affect their audit plans. Instead of auditing by clause, your organisation may decide to inspect by functional expanse.
Develop a competence and preparation schedule for the internal auditors, ensure this is in your projection plan.
Learn more nearly internal auditing
xi. Implement the QMS
Monitor and mensurate procedure performance and start internal audits.
Our Quality Manual Template comes complete with all necessary Forms, including:
- Master Certificate & Record Index
- Document Outcome Sheet
- Document Change Request
- Take a chance & Opportunity Annals
- SWOT Analysis Template
- PESTLE Analysis Template
- Competency Review
- Preparation Attendance
- Training Evaluation
- Controlled Equipment Log
- Scale Log
- Software Validation Log
- Requirements Review Checklist
- Design Alter Request
- Design Change Request Log
- Canonical Supplier Alphabetize
- Supplier Evaluation
- Receiving Inspection Log
- Non-conformance Written report
- Concession Request
- Concession Request Log
- Corrective Action Report
- Customer Satisfaction Survey
- Customer Feedback Log
- Internal Audit Study
- Internal Inspect Feedback
- Management Review Agenda & Minutes
- View sample Form
12. Select Certification Body
Select your Certification Body and agree the Scope of Registration.
Why Reinvent the Cycle?
Our ISO 9001 Project Plan Template is proven to work.
13. Refine the QMS
Make whatsoever necessary changes to the QMS and Quality Manual. Near certification bodies wish to see at least three months of record history.

14. Management Review
The Management Review is your last cheque to ensure that everyone is happy therefore you lot should review the business, not just "quality". This vital footstep is traditionally represented past a minimal, typically annual, senior management review of the QMS.
ISO 9001:2015 requires that the review generates conclusion on key matters such as process improvement, resources allocation, product comeback driven by customer requirements, and the establishment of new improvement objectives.
Bearing in heed the importance of these sorts of topics, it is best not to hold a separate review, knowing that this sends signals to people in the organization that quality is outside the normal activities of management.
Summary of Management Review actions and benefits
- Define 'quality' in the form of objectives to help internal advice of what is to be accomplished (production and service requirements, process effectiveness and efficiency, customer perception etc.)
- Show that the business is central to the organization: use your normal business language, not 'quality' or ISO 9001 terms.
- Produce a elementary top-level, "large picture" of your business processes to show how the arrangement improves results past focusing on the comeback of processes.
- Demonstrate your commitment to continual improvement by focusing on the next improvement and by taking information technology seriously.
- Show that the 'quality' arroyo is becoming instituted by integrating reviews into normal management cycles.
- Ensure that records are turned visibly into management information then that people keeping them empathise their importance.
15. Implement any System Changes
Implement whatsoever changes to the Quality Management System that might take arisen from the outputs of previous steps.
16. Certification Torso preliminaries
The Documentation Audit
The Documentation Audit is a desk-bound-based practice carried out by auditors either in their own offices or at the visitor being audited. The inspect is restricted to the quality manual and related systems. Its' aim is to ensure that the documentation addresses the elements of ISO 9001. If the auditors identify major gaps in your QMS, at that place is fiddling point in proceeding with the assessment until these are rectified.
The Pre-Assessment Audit
The Pre-Assessment Audit (optional, also know as the ISO 9001 Pre Accreditation Audit) is a mock audit in preparation for the real thing. A pre-assessment identifies problems and enables the visitor to benefit from the advice of the auditor on how to eliminate those issues.
Y'all may pay extra, but information technology is often worthwhile to accommodate a pre-assessment visit. Make sure you sympathize and concord any non-conformances. If not, ask for a 2d stance.
Accommodate certification engagement.

17. Certification Day
The Implementation Audit
This is an on-site audit. It involves a systematic examination of the company'south QMS against the ISO 9001 standard.
The emphasis is placed on finding objective evidence that demonstrates it has been implemented effectively and that any procedures are beingness followed.
The beginning areas generally examined are management commitment (quality policy and advice), management reviews, corrective actions taken, quality objectives, continual improvement and changes fabricated as the result of the pre-cess audit.
Make sure you lot sympathize and agree any non-conformances. If not, enquire for a 2d stance.
Jump Start Your ISO Project
Our ISO 9001 Projection Plan Template is proven to work.
18. Maintain and improve your QMS
Processes can always be more efficient and effective, fifty-fifty when they're producing conforming products. The aim of a continual improvement programme is to increase the odds of satisfying customers past identifying areas needing improvement. After setting improvement objectives, an organization searches for possible solutions, selects and implements the appropriate one and evaluates results to ostend that objectives are met.
Useful external links
- Choosing a certification body - www.iso.org
- Choosing an accreditation service - www.ukas.org
ISO 9001 Implementation
- What are the steps for ISO certification? (Overview)
- How Hard is it to Become ISO 9001 Certification?
- How to Implement ISO 9001 (In 8 Steps)
- ISO 9001 Project Plan
- ISO 9001 Implementation Guide (detailed, 27 Steps)
- How to Utilize for ISO 9001 Certification in five Simple Steps
Source: https://www.iso-9001-checklist.co.uk/iso-9001-project-plan.htm
Post a Comment for "Engineer Review Customer Requirements for System Implementation Project Plan"